Red cabbage indicator is often used as an introduction to pH and indicators, but did you know you can make an indicator from blackberries? Blackberries contain anthocyanins, which are pigments that change colour depending on pH. Anthocyanins are a natural indicator.
An indicator is a substance that changes colour when added to solutions of a different pH.
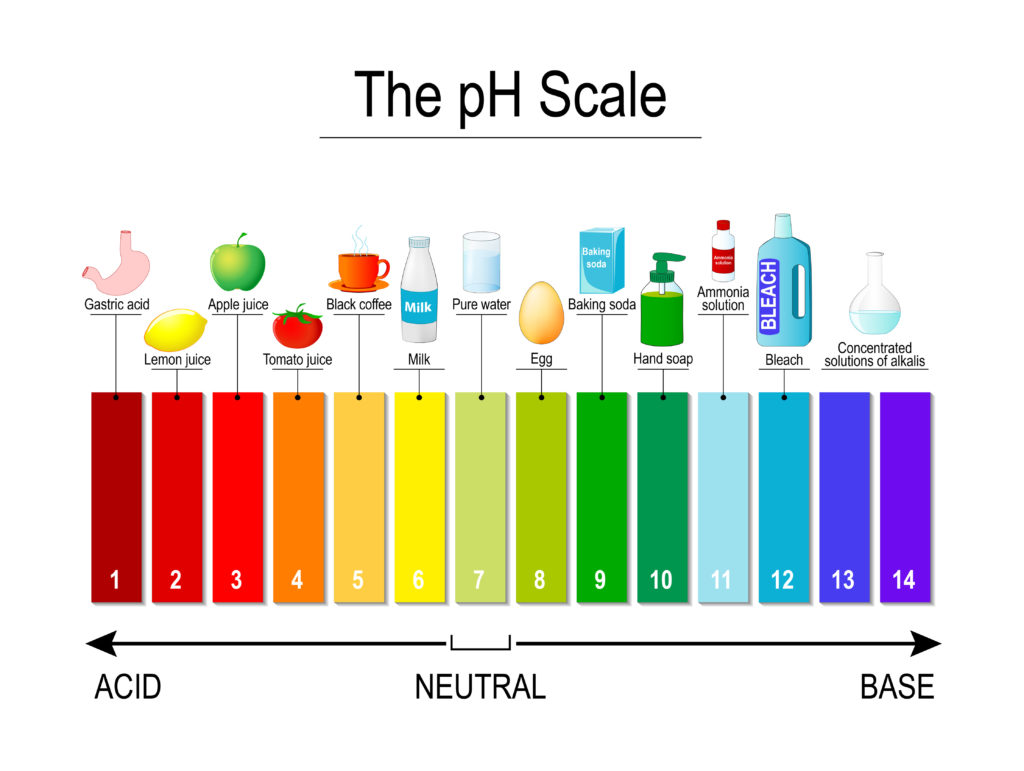
The pH scale measures the acidity of a substance. The colours in the image below are from Universal Indicator, which shows how acidic or alkaline a substance is. Most other indicators just show whether a solution is acidic or alkaline, not the strength.
How to make a pH indicator with blackberries
Materials
Water
Pan
Heat source
Spoon
Vinegar
Baking Soda
Toothpaste
Blackberries
Containers for testing
Instructions
Collect a handful of blackberries and add them to about 200ml of water.
Heat gently in a pan until the water changes colour.
Leave to cool.
Carefully sieve the mixture to remove the crushed blackberries, and save the liquid. This is your blackberry indicator.
Pour a small amount of the indicator into a container and add a small amount of one of the test substances.
Repeat for each substance.

The blackberry indicator is fairly basic, but a good introduction to pH, acids, and alkalis.
Make Blackberry Indicator Paper
If you have some filter paper or kitchen towel, blackberry indicator can be used to make indicator paper!
Dip the filter paper or kitchen towel in the indicator and leave it to dry.
Use a pipette to drop a small amount of each test substance onto the paper and observe the colour change.
The indicator paper will turn purple in the presence of an alkali and pink in the presence of an acid.

Extension task
Try using just the juice from blackberries without adding boiling water.
Test other fruits that contain anthocyanins, such as blueberries and blackcurrants.
Make an indicator from red cabbage and test the same substances.

Last Updated on September 3, 2025 by Emma Vanstone




Leave a Reply