Today’s post is part of my United States of Science series. The idea is you print a map of the USA and colour each state as you learn about it with a fun science activity.
New York
New York City in the state of New York is the most populated city in the whole of the United States, with a population of over 8.5 million people.
The Statue of Liberty is just one of the iconic landmarks in New York City. It’s made of copper, which has oxidised naturally to form a green patina coating which actually protects the copper underneath.
It took about 20 years for the Statue of Liberty to change from copper coloured to green!

Why is the Statue of Liberty Green?

Some simple copper chemistry will help us to find out. Copper coins can be cleaned with vinegar and salt.
What you need to clean coins
Vinegar
Salt
Small bowl
Copper coins
How to clean coins
1. Put about a teaspoon of salt into a bowl
2. Add about 50ml of vinegar and stir to dissolve the salt.
3. Leave the coins in the vinegar and salt solution for 5 minutes.
5. Take them out and compare them to other dull copper coins!
You now have sparkling copper treasure!
Why does vinegar clean coins?
The reason copper coins don’t stay shiny is that the copper reacts with oxygen in the air to form copper oxide, which is a dull greenish-grey colour.
When you mix salt (sodium chloride) with vinegar (acetic acid), sodium acetate and hydrogen chloride form. Hydrogen chloride is an acid which works well at rapidly cleaning the surface of the copper coin, leaving it beautifully shiny and removing the oxide.
If the coin is exposed to the air again, it will quickly react with oxygen to form the dull greenish copper oxide layer.
The Statue of Liberty is coated in a thin layer of copper which has turned green due to reactions with air and water.
Now you know why the Statue of Liberty is green!
This activity would be great for a school science fair project.
If you enjoyed this activity, don’t forget I have lots more fun Chemistry science experiments for kids of all ages!
How else can you clean coins?
Anything acidic will clean coins, and if it’s acidic and salty, even better!
A fun way to extend this investigation is to try to clean copper coins with other substances. Ketchup, coke and lemon juice are all good things to try first!
Do not eat or drink anything you’ve used to clean coins.
New York Facts
The Statue of Liberty gets struck by lightning multiple times a year.
New York is nicknamed The Empire State.
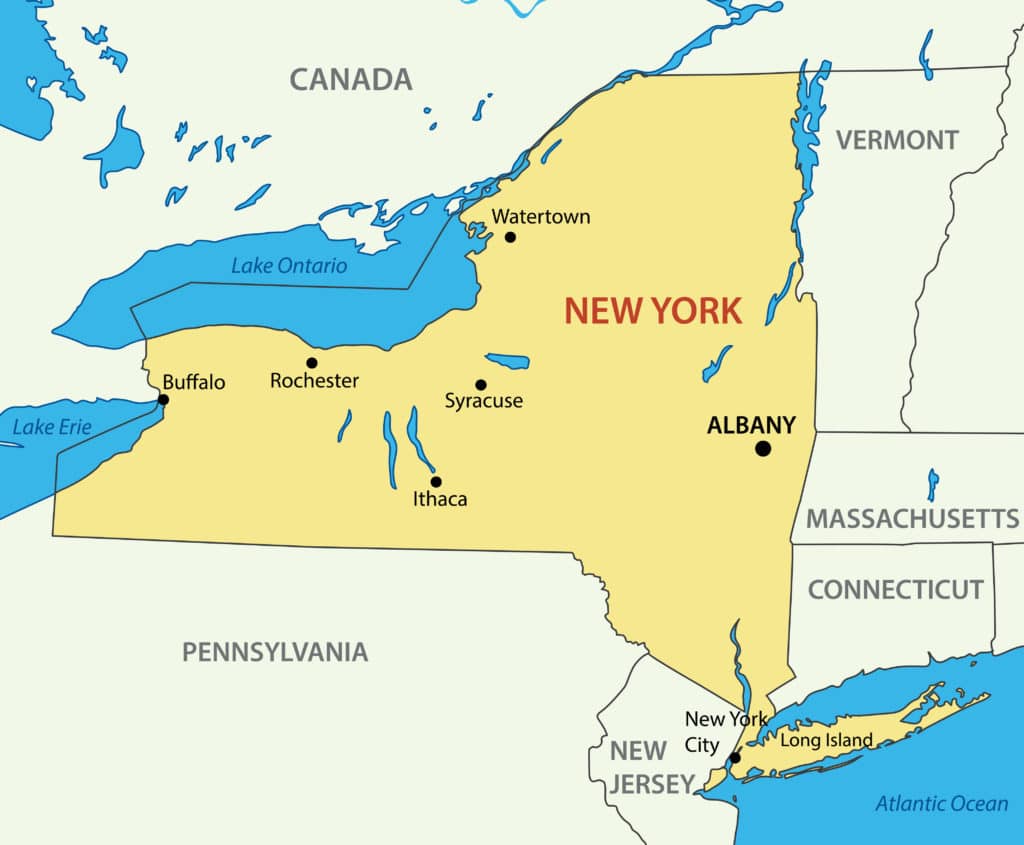
The capital city of New York is Albany, not New York City!
New York is bordered by Pennsylvania, New Jersey, Connecticut, Massachusetts, Vermont and Canada!
Niagara Falls borders New York and Canada.


Last Updated on February 23, 2024 by Emma Vanstone





Ah, nice good test.
i didn’t know the statue of liberty was copper!
Just covered in a thin layer!
What a wonderful hands on way to learn about the statue of liberty, I will be adding this to out United States projects for sure!! Thanks so much for sharing!! Have a wonderful weekend!
and you!
Is it chemical or physical change?
This would be a chemical change.
NaCl+ CH3CH2OH is not possible…:D
I was wondering why the statue of liberty turned green! Thanks for the cleaning tip!