This dissolving candy canes activity is a great science activity for before Christmas when the festivities are in full swing or after Christmas to use up any leftover candy canes.
The idea is to place candy canes in different liquids to find where they melt the fastest. We used hot and cold water and vinegar, but different hot chocolate temperatures would also work well.

Dissolving candy canes is an excellent investigation for learning how to set up a fair test! This means you need to think about which variables you change and which you keep the same.
Controlled variables are things you keep the same. In this investigation, the controlled variables are:
- size of candy cane
- time the candy cane is in the liquid
- amount of liquid used
The independent variable is the thing you change. In this experiment, the independent variable is the liquid in which the candy canes are sat.
The dependent variable is the thing you measure. In this investigation, the time it takes for the candy cane to dissolve is measured.
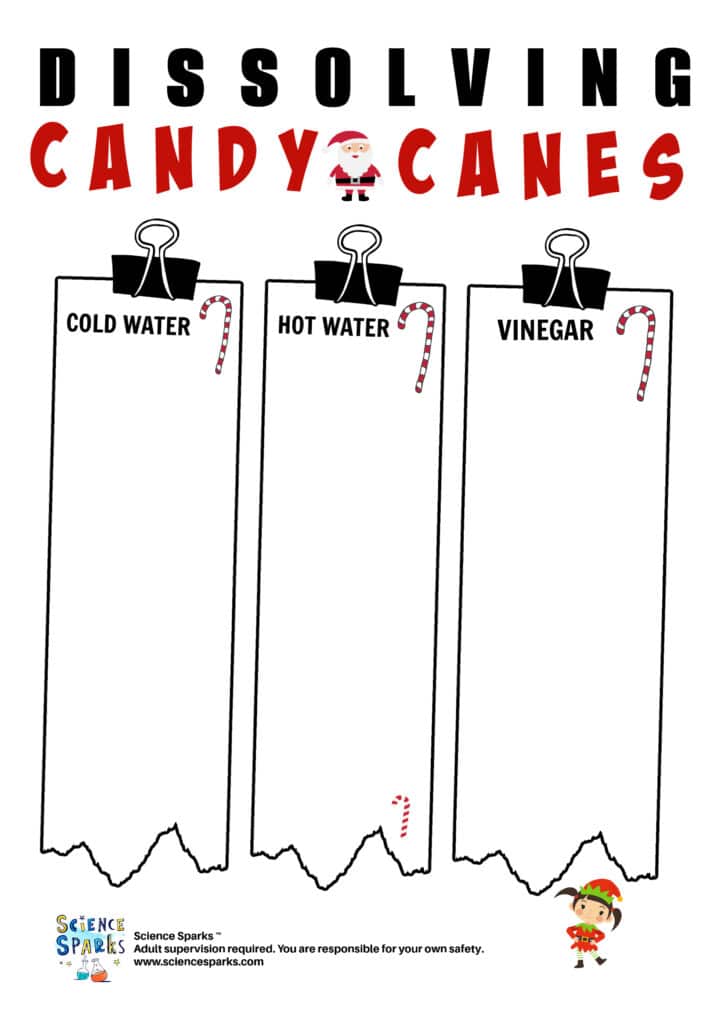
Candy Cane Experiment – dissolving candy canes
You’ll need the following:
Three containers of the same size
Vinegar
Cold Water
Hot Water
Method
Set up your containers. Take care with the hot water.
Add a candy cane to each container the same way up.
Observe each candy cane at 5-minute intervals ( can you design a table to record your observations? )
Results
The photo below shows our final results after 20 minutes.
Vinegar is on the left, cold water is in the middle, and hot water is on the right.

You can see that the vinegar completely dissolved the submerged candy cane, the cold water just dissolved the outer layer and the hot water dissolved past the outer layer making the submerged section break off.
The thing we found most interesting was that the hot water turned red, and the vinegar and cold water turned grey.
Record the results on the handy dissolving candy canes instruction sheet below.
More Candy Cane Experiments
Find out how strong a candy cane is by hanging decorations from the end.
Inspiration Laboratories has a great candy cane activity using different water temperatures that would be great to try too.

More Christmas Science Experiments
Try one of my festive candy experiments! These include building marshmallow snowmen, making sugar crystal lollies and making delicious peppermint candies.
Try these fun Christmas Lava Lamps. We made ours snowman and reindeer themed, but you could make anything you wanted! How about an elf lava lamp?

Set up a Fizzy Elf Lab and determine what happens when baking soda and vinegar react.

How about making a fun Frosty the Snowman? Making frost on a can is a brilliant way to find out how salt makes a mixture of water and ice extra cold!
I also have a FREE Christmas Science eBook full of easy Christmas science experiments you might like!

Last Updated on December 12, 2022 by Emma Vanstone


Your candy canes have green on them. The cold jar candy cane looks like thick red with thin green stripes while the others more even. The mixing of more green than red may be the cause of the grey water.
Also, the cold may not dissolve the green as quickly. I cannot tell if there is still green on the candy cane still in the cold water. If this were the case, then the cold would have more red.
Just my hypothesis. 😀